Cobalt »
PDB 1a0c-1e1c »
1e1c »
Cobalt in PDB 1e1c: Methylmalonyl-Coa Mutase H244A Mutant
Enzymatic activity of Methylmalonyl-Coa Mutase H244A Mutant
All present enzymatic activity of Methylmalonyl-Coa Mutase H244A Mutant:
5.4.99.2;
5.4.99.2;
Protein crystallography data
The structure of Methylmalonyl-Coa Mutase H244A Mutant, PDB code: 1e1c
was solved by
P.R.Evans,
N.H.Thoma,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 29.20 / 2.62 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 125.398, 161.829, 166.949, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 20.7 / 28.3 |
Cobalt Binding Sites:
The binding sites of Cobalt atom in the Methylmalonyl-Coa Mutase H244A Mutant
(pdb code 1e1c). This binding sites where shown within
5.0 Angstroms radius around Cobalt atom.
In total 2 binding sites of Cobalt where determined in the Methylmalonyl-Coa Mutase H244A Mutant, PDB code: 1e1c:
Jump to Cobalt binding site number: 1; 2;
In total 2 binding sites of Cobalt where determined in the Methylmalonyl-Coa Mutase H244A Mutant, PDB code: 1e1c:
Jump to Cobalt binding site number: 1; 2;
Cobalt binding site 1 out of 2 in 1e1c
Go back to
Cobalt binding site 1 out
of 2 in the Methylmalonyl-Coa Mutase H244A Mutant
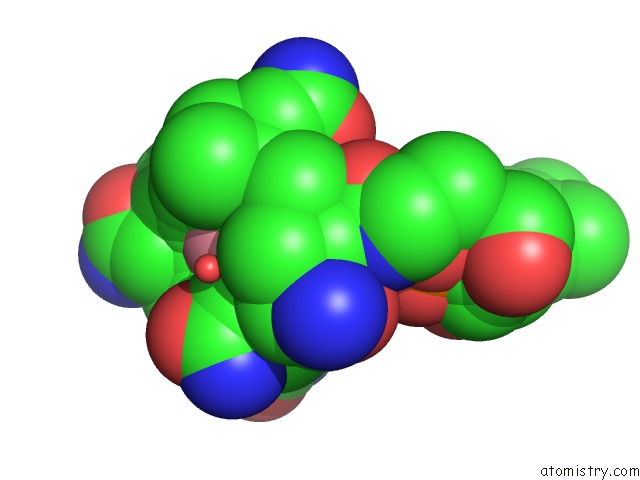
Mono view
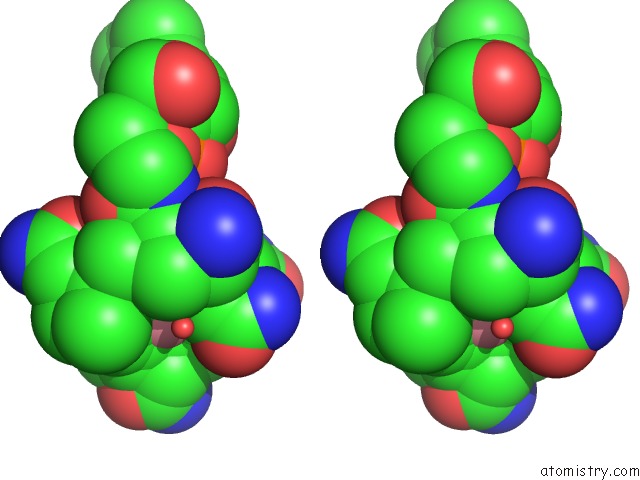
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 1 of Methylmalonyl-Coa Mutase H244A Mutant within 5.0Å range:
|
Cobalt binding site 2 out of 2 in 1e1c
Go back to
Cobalt binding site 2 out
of 2 in the Methylmalonyl-Coa Mutase H244A Mutant
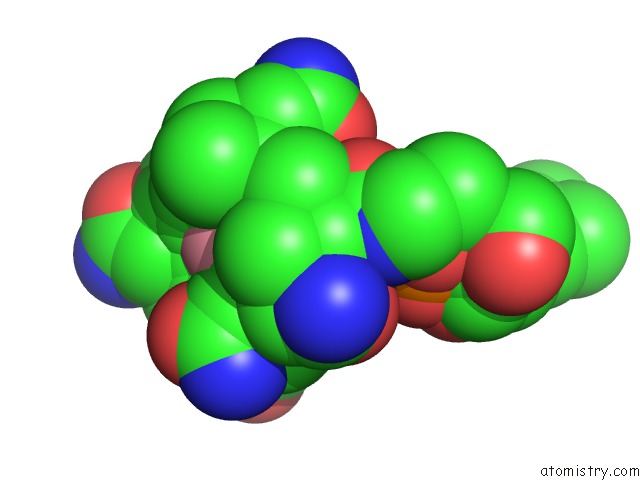
Mono view
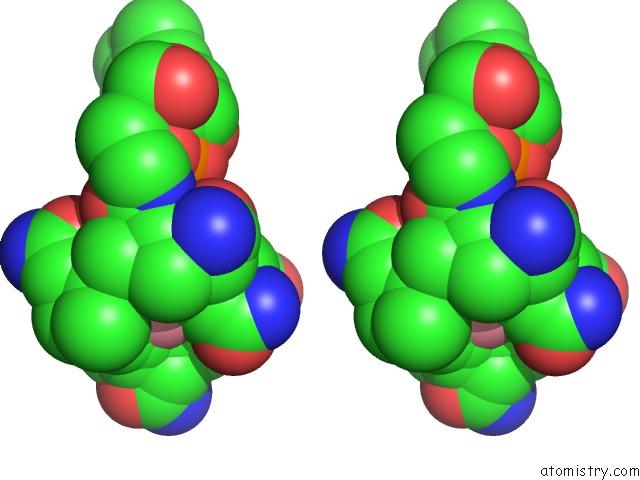
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 2 of Methylmalonyl-Coa Mutase H244A Mutant within 5.0Å range:
|
Reference:
N.H.Thoma,
P.R.Evans,
P.F.Leadlay.
Protection of Radical Intermediates at the Active Site of Adenosylcobalamin-Dependent Methylmalonyl-Coa Mutase Biochemistry V. 39 9213 2000.
ISSN: ISSN 0006-2960
PubMed: 10924114
DOI: 10.1021/BI0004302
Page generated: Sun Jul 13 17:27:26 2025
ISSN: ISSN 0006-2960
PubMed: 10924114
DOI: 10.1021/BI0004302
Last articles
Cu in 4EV2Cu in 4F2E
Cu in 4ENZ
Cu in 4EJX
Cu in 4EF3
Cu in 4EIR
Cu in 4EIS
Cu in 4E9X
Cu in 4E9Y
Cu in 4E9V