Cobalt »
PDB 2djl-2g6p »
2dxc »
Cobalt in PDB 2dxc: Recombinant Thiocyanate Hydrolase, Fully-Matured Form
Enzymatic activity of Recombinant Thiocyanate Hydrolase, Fully-Matured Form
All present enzymatic activity of Recombinant Thiocyanate Hydrolase, Fully-Matured Form:
3.5.5.8;
3.5.5.8;
Protein crystallography data
The structure of Recombinant Thiocyanate Hydrolase, Fully-Matured Form, PDB code: 2dxc
was solved by
T.Arakawa,
Y.Kawano,
Y.Katayama,
M.Yohda,
M.Odaka,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 41.85 / 1.90 |
| Space group | P 21 21 21 |
| Cell size a, b, c (Å), α, β, γ (°) | 114.874, 170.531, 175.152, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 17.1 / 19.3 |
Cobalt Binding Sites:
The binding sites of Cobalt atom in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form
(pdb code 2dxc). This binding sites where shown within
5.0 Angstroms radius around Cobalt atom.
In total 4 binding sites of Cobalt where determined in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form, PDB code: 2dxc:
Jump to Cobalt binding site number: 1; 2; 3; 4;
In total 4 binding sites of Cobalt where determined in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form, PDB code: 2dxc:
Jump to Cobalt binding site number: 1; 2; 3; 4;
Cobalt binding site 1 out of 4 in 2dxc
Go back to
Cobalt binding site 1 out
of 4 in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form
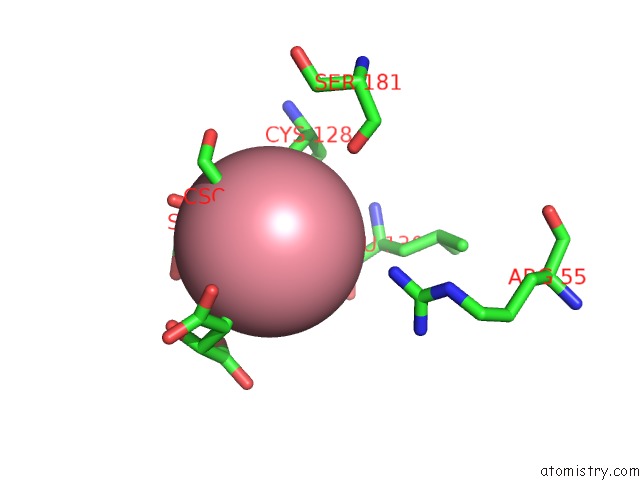
Mono view
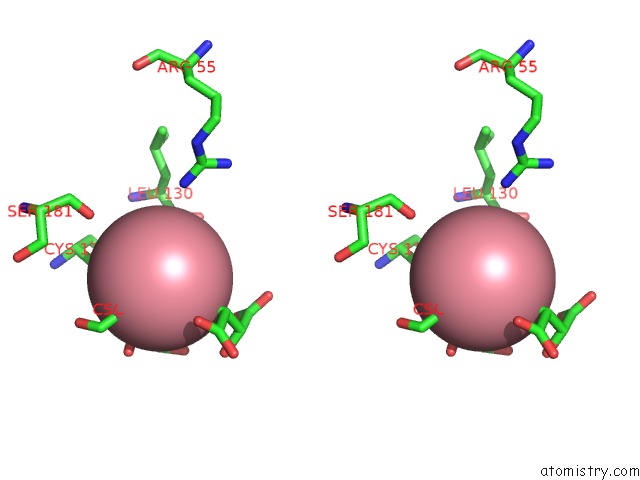
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 1 of Recombinant Thiocyanate Hydrolase, Fully-Matured Form within 5.0Å range:
|
Cobalt binding site 2 out of 4 in 2dxc
Go back to
Cobalt binding site 2 out
of 4 in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form
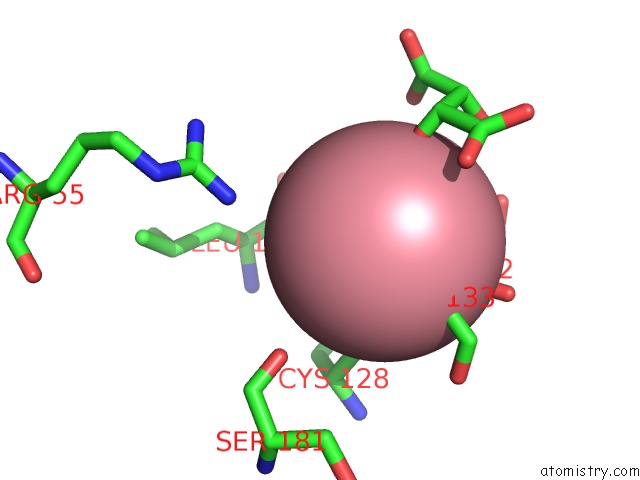
Mono view
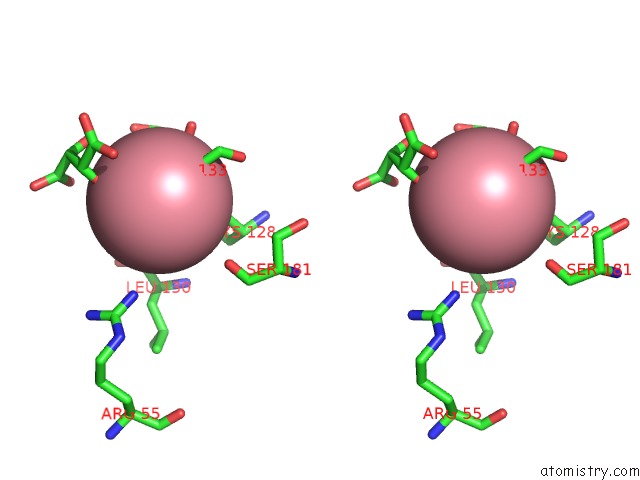
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 2 of Recombinant Thiocyanate Hydrolase, Fully-Matured Form within 5.0Å range:
|
Cobalt binding site 3 out of 4 in 2dxc
Go back to
Cobalt binding site 3 out
of 4 in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form
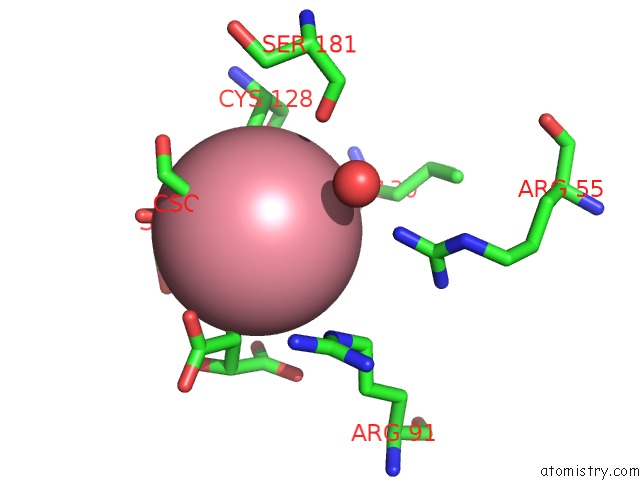
Mono view
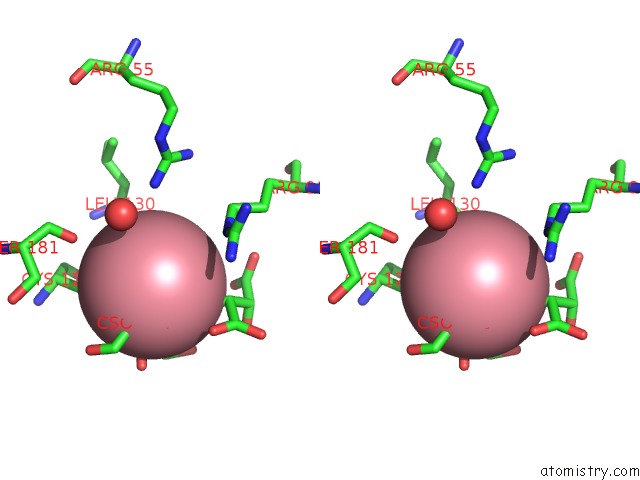
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 3 of Recombinant Thiocyanate Hydrolase, Fully-Matured Form within 5.0Å range:
|
Cobalt binding site 4 out of 4 in 2dxc
Go back to
Cobalt binding site 4 out
of 4 in the Recombinant Thiocyanate Hydrolase, Fully-Matured Form
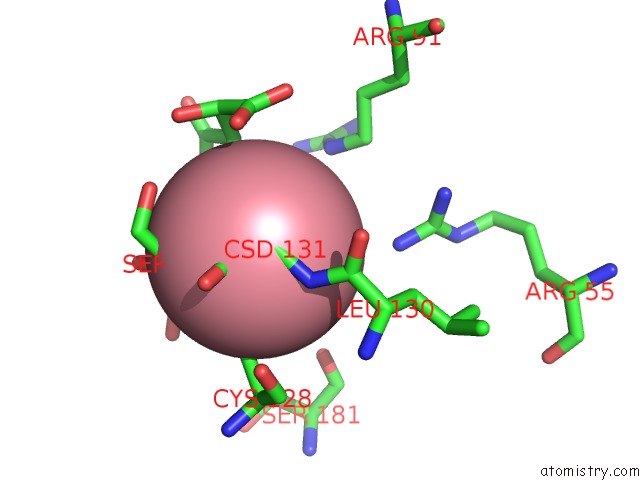
Mono view
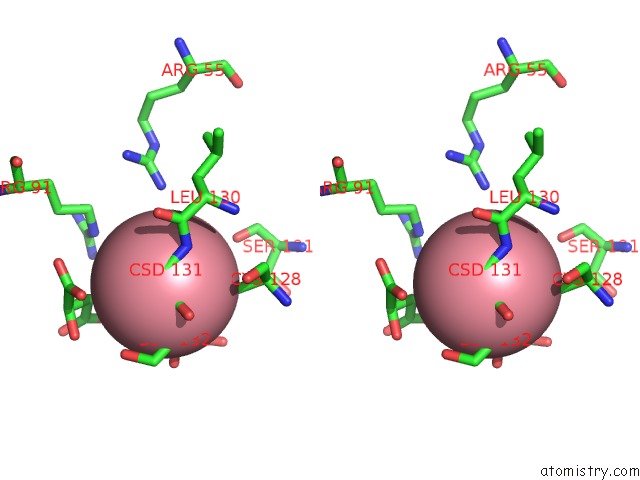
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 4 of Recombinant Thiocyanate Hydrolase, Fully-Matured Form within 5.0Å range:
|
Reference:
T.Arakawa,
Y.Kawano,
Y.Katayama,
H.Nakayama,
N.Dohmae,
M.Yohda,
M.Odaka.
Structural Basis For Catalytic Activation of Thiocyanate Hydrolase Involving Metal-Ligated Cysteine Modification J.Am.Chem.Soc. V. 131 14838 2009.
ISSN: ISSN 0002-7863
PubMed: 19785438
DOI: 10.1021/JA903979S
Page generated: Sun Jul 13 18:08:22 2025
ISSN: ISSN 0002-7863
PubMed: 19785438
DOI: 10.1021/JA903979S
Last articles
Cu in 3CE1Cu in 3BVD
Cu in 3CDZ
Cu in 3C75
Cu in 3BKT
Cu in 3BQV
Cu in 3B1J
Cu in 3AZU
Cu in 3AX0
Cu in 3AWX