Cobalt »
PDB 5d6f-5ikv »
5fdq »
Cobalt in PDB 5fdq: Murine Cox-2 S530T Mutant
Enzymatic activity of Murine Cox-2 S530T Mutant
All present enzymatic activity of Murine Cox-2 S530T Mutant:
1.14.99.1;
1.14.99.1;
Protein crystallography data
The structure of Murine Cox-2 S530T Mutant, PDB code: 5fdq
was solved by
M.J.Lucido,
B.J.Orlando,
M.G.Malkowski,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 19.96 / 1.90 |
| Space group | I 2 2 2 |
| Cell size a, b, c (Å), α, β, γ (°) | 120.163, 132.500, 180.488, 90.00, 90.00, 90.00 |
| R / Rfree (%) | 15.3 / 19.1 |
Cobalt Binding Sites:
The binding sites of Cobalt atom in the Murine Cox-2 S530T Mutant
(pdb code 5fdq). This binding sites where shown within
5.0 Angstroms radius around Cobalt atom.
In total 2 binding sites of Cobalt where determined in the Murine Cox-2 S530T Mutant, PDB code: 5fdq:
Jump to Cobalt binding site number: 1; 2;
In total 2 binding sites of Cobalt where determined in the Murine Cox-2 S530T Mutant, PDB code: 5fdq:
Jump to Cobalt binding site number: 1; 2;
Cobalt binding site 1 out of 2 in 5fdq
Go back to
Cobalt binding site 1 out
of 2 in the Murine Cox-2 S530T Mutant
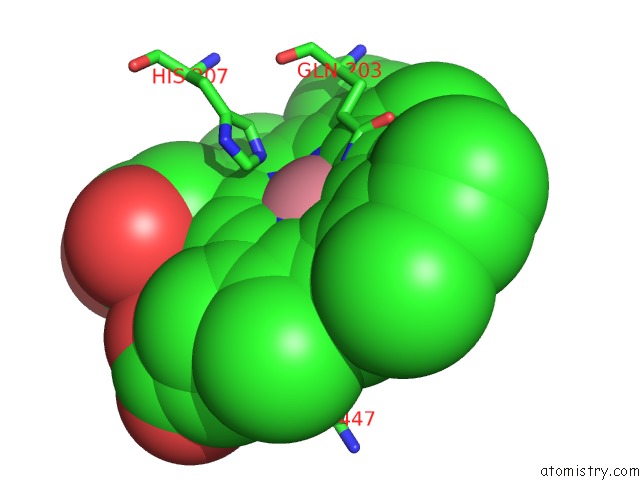
Mono view
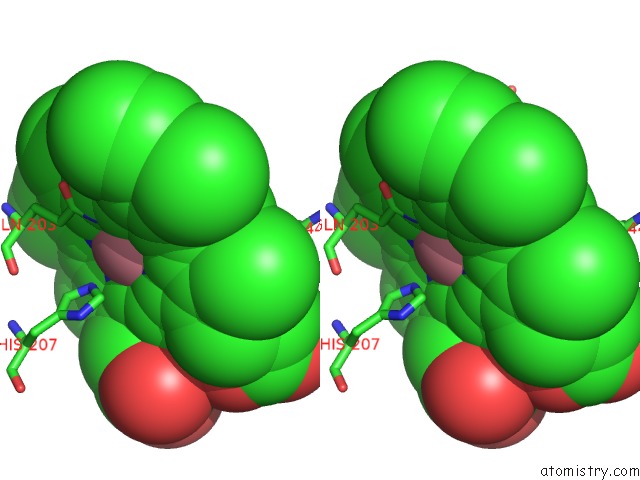
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 1 of Murine Cox-2 S530T Mutant within 5.0Å range:
|
Cobalt binding site 2 out of 2 in 5fdq
Go back to
Cobalt binding site 2 out
of 2 in the Murine Cox-2 S530T Mutant
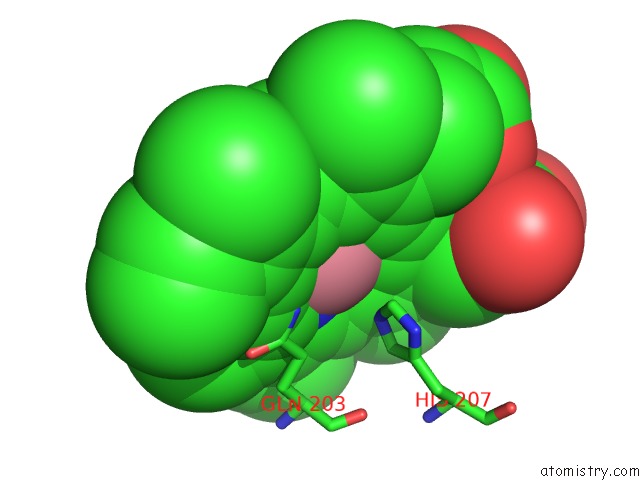
Mono view
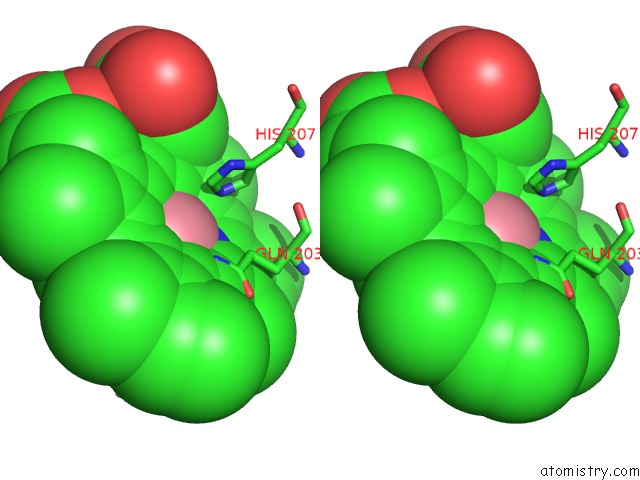
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 2 of Murine Cox-2 S530T Mutant within 5.0Å range:
|
Reference:
M.J.Lucido,
B.J.Orlando,
A.J.Vecchio,
M.G.Malkowski.
Crystal Structure of Aspirin-Acetylated Human Cyclooxygenase-2: Insight Into the Formation of Products with Reversed Stereochemistry. Biochemistry V. 55 1226 2016.
ISSN: ISSN 0006-2960
PubMed: 26859324
DOI: 10.1021/ACS.BIOCHEM.5B01378
Page generated: Tue Jul 30 17:53:02 2024
ISSN: ISSN 0006-2960
PubMed: 26859324
DOI: 10.1021/ACS.BIOCHEM.5B01378
Last articles
As in 1P6NAs in 1P13
As in 1P6M
As in 1OKG
As in 1P6L
As in 1OB1
As in 1NLX
As in 1OAR
As in 1NW2
As in 1NQ2