Cobalt »
PDB 6kgh-6oxc »
6nw5 »
Cobalt in PDB 6nw5: Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
Protein crystallography data
The structure of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors, PDB code: 6nw5
was solved by
R.Dutoit,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 47.60 / 1.70 |
| Space group | H 3 |
| Cell size a, b, c (Å), α, β, γ (°) | 131.152, 131.152, 285.610, 90.00, 90.00, 120.00 |
| R / Rfree (%) | 14.3 / 16.4 |
Other elements in 6nw5:
The structure of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors also contains other interesting chemical elements:
| Zinc | (Zn) | 4 atoms |
Cobalt Binding Sites:
The binding sites of Cobalt atom in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
(pdb code 6nw5). This binding sites where shown within
5.0 Angstroms radius around Cobalt atom.
In total 4 binding sites of Cobalt where determined in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors, PDB code: 6nw5:
Jump to Cobalt binding site number: 1; 2; 3; 4;
In total 4 binding sites of Cobalt where determined in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors, PDB code: 6nw5:
Jump to Cobalt binding site number: 1; 2; 3; 4;
Cobalt binding site 1 out of 4 in 6nw5
Go back to
Cobalt binding site 1 out
of 4 in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
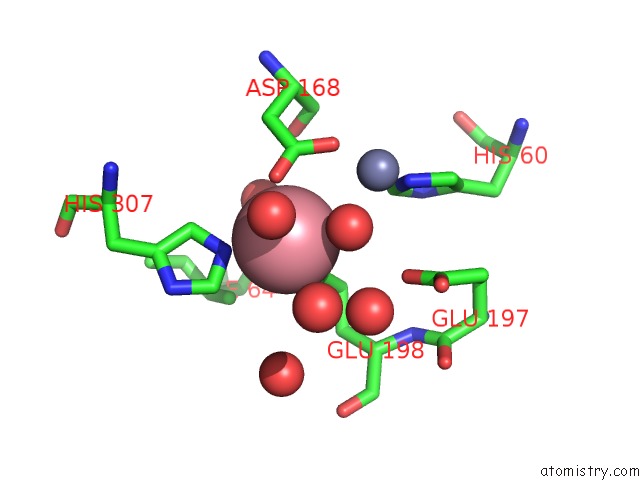
Mono view
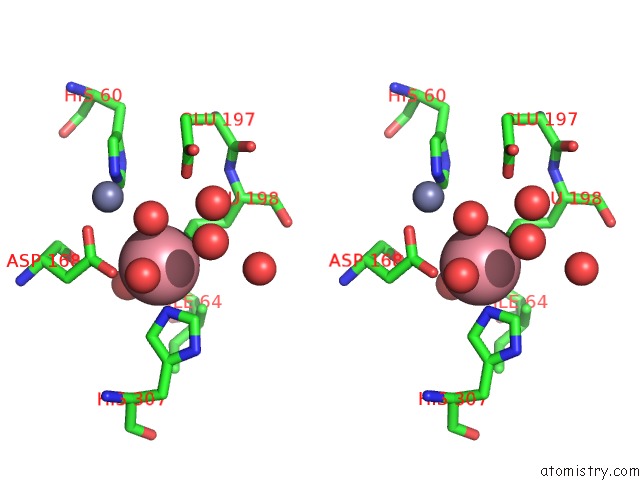
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 1 of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors within 5.0Å range:
|
Cobalt binding site 2 out of 4 in 6nw5
Go back to
Cobalt binding site 2 out
of 4 in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
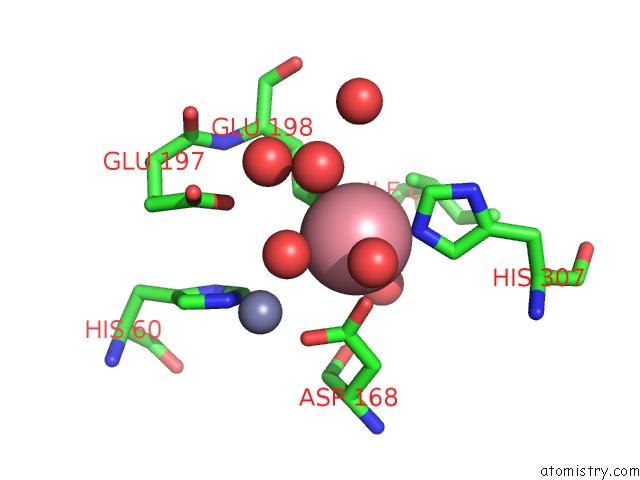
Mono view
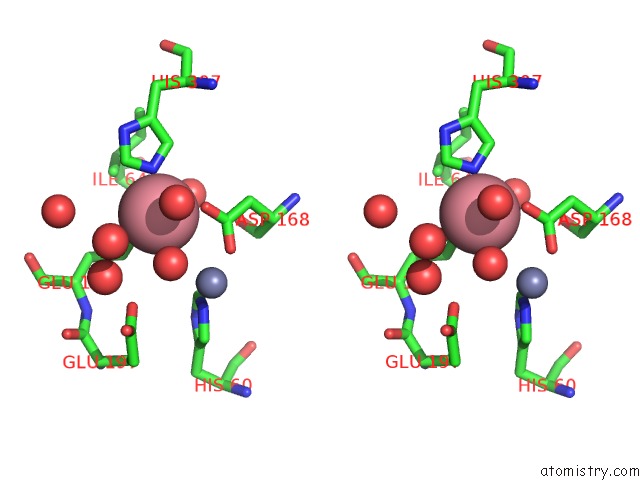
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 2 of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors within 5.0Å range:
|
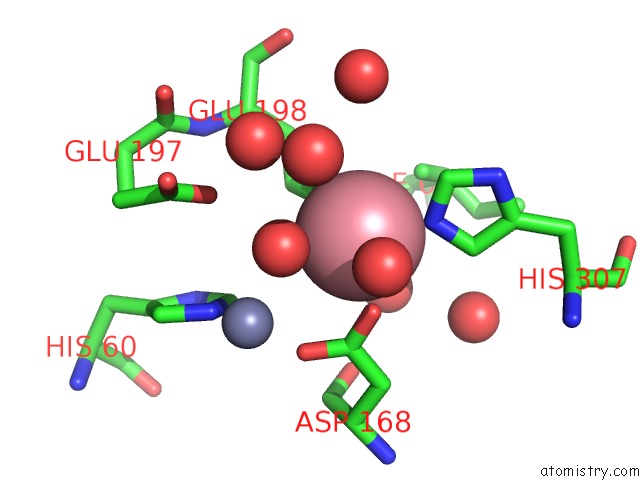
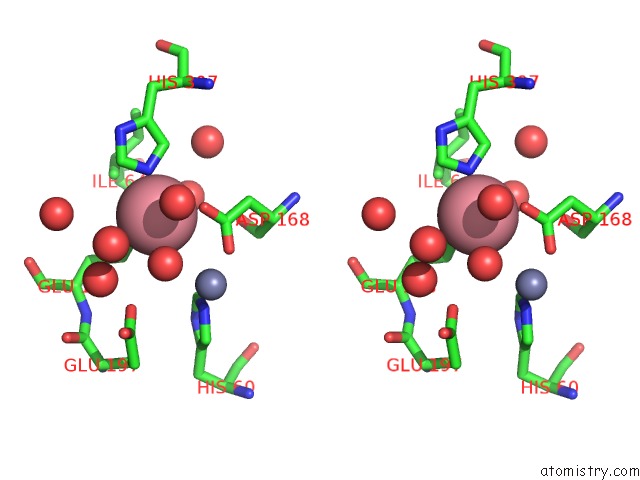
Cobalt binding site 3 out of 4 in 6nw5
Go back to
Cobalt binding site 3 out
of 4 in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 3 of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors within 5.0Å range:
|
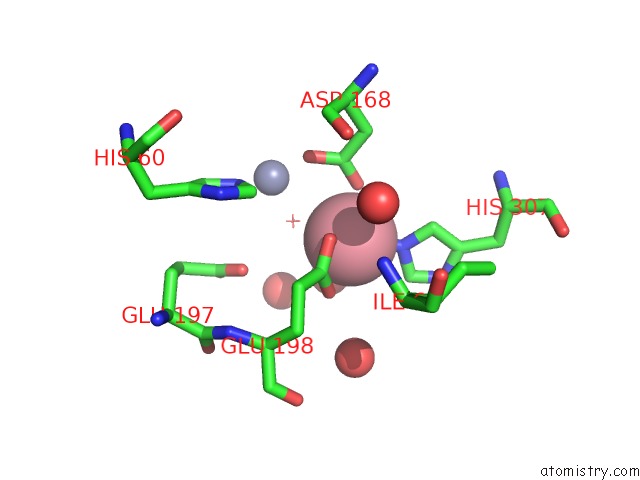
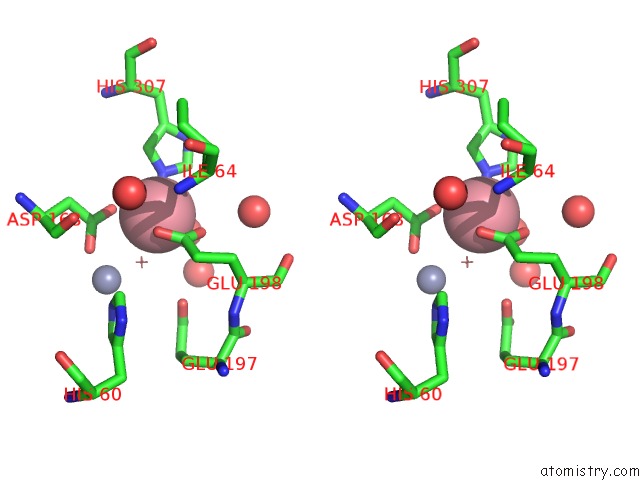
Cobalt binding site 4 out of 4 in 6nw5
Go back to
Cobalt binding site 4 out
of 4 in the Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors
Mono view
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 4 of Crystal Structure of TMPEP1050 Aminopeptidase with Its Metal Cofactors within 5.0Å range:
|
Reference:
R.Dutoit,
T.Van Gompel,
N.Brandt,
D.Van Elder,
J.Van Dyck,
F.Sobott,
L.Droogmans.
How Metal Cofactors Drive Dimer-Dodecamer Transition of the M42 Aminopeptidase TMPEP1050 Ofthermotoga Maritima. J.Biol.Chem. V. 294 17777 2019.
ISSN: ESSN 1083-351X
PubMed: 31611236
DOI: 10.1074/JBC.RA119.009281
Page generated: Sun Jul 13 21:10:36 2025
ISSN: ESSN 1083-351X
PubMed: 31611236
DOI: 10.1074/JBC.RA119.009281
Last articles
Cu in 6I3JCu in 6HWH
Cu in 6HU9
Cu in 6HBE
Cu in 6I1J
Cu in 6HY8
Cu in 6H5Y
Cu in 6HQI
Cu in 6G5M
Cu in 6HAQ