Cobalt »
PDB 8im2-8sfb »
8qex »
Cobalt in PDB 8qex: Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat)
Protein crystallography data
The structure of Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat), PDB code: 8qex
was solved by
R.P.Jakob,
D.Chen,
T.R.Ward,
with X-Ray Crystallography technique. A brief refinement statistics is given in the table below:
| Resolution Low / High (Å) | 45.25 / 1.90 |
| Space group | I 1 2 1 |
| Cell size a, b, c (Å), α, β, γ (°) | 48.456, 80.534, 78.766, 90, 103.92, 90 |
| R / Rfree (%) | 22.9 / 25.4 |
Cobalt Binding Sites:
The binding sites of Cobalt atom in the Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat)
(pdb code 8qex). This binding sites where shown within
5.0 Angstroms radius around Cobalt atom.
In total 2 binding sites of Cobalt where determined in the Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat), PDB code: 8qex:
Jump to Cobalt binding site number: 1; 2;
In total 2 binding sites of Cobalt where determined in the Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat), PDB code: 8qex:
Jump to Cobalt binding site number: 1; 2;
Cobalt binding site 1 out of 2 in 8qex
Go back to
Cobalt binding site 1 out
of 2 in the Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat)
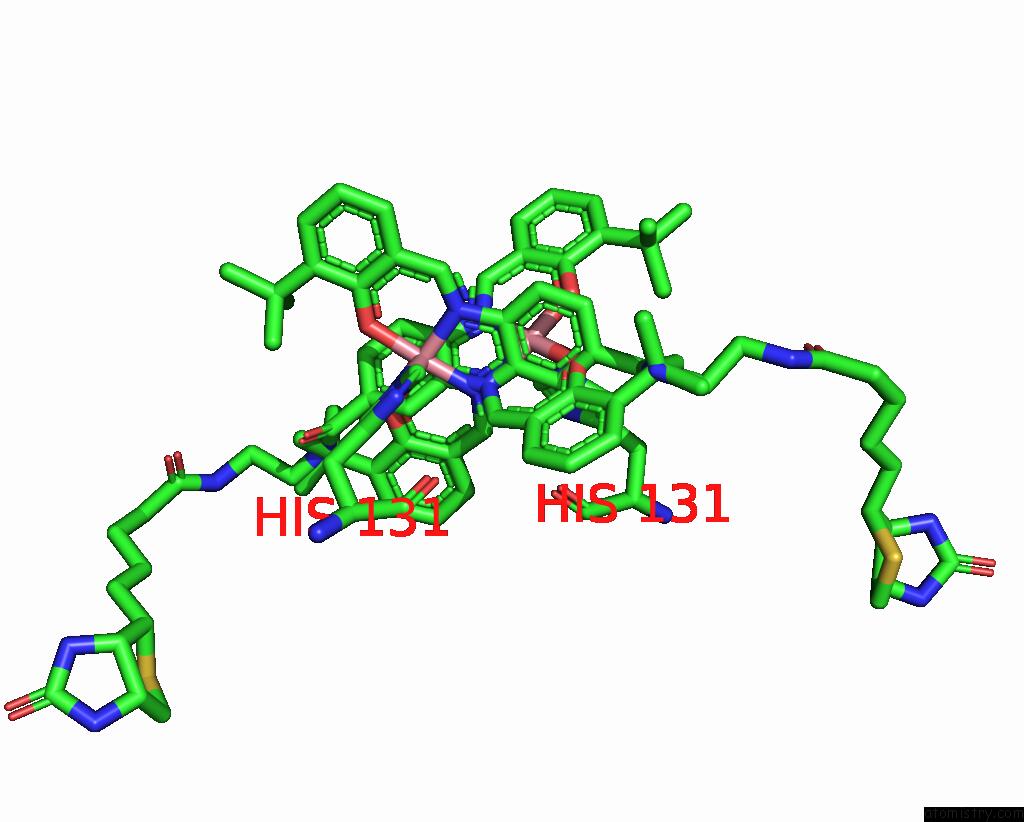
Mono view
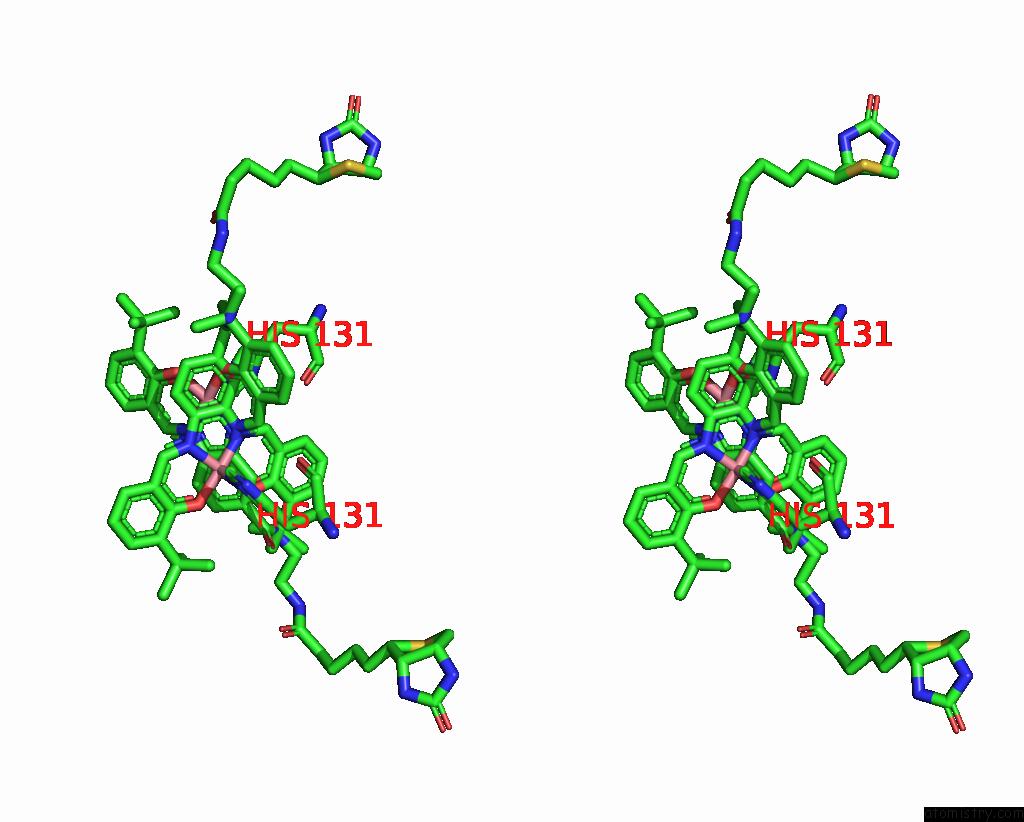
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 1 of Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat) within 5.0Å range:
|
Cobalt binding site 2 out of 2 in 8qex
Go back to
Cobalt binding site 2 out
of 2 in the Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat)
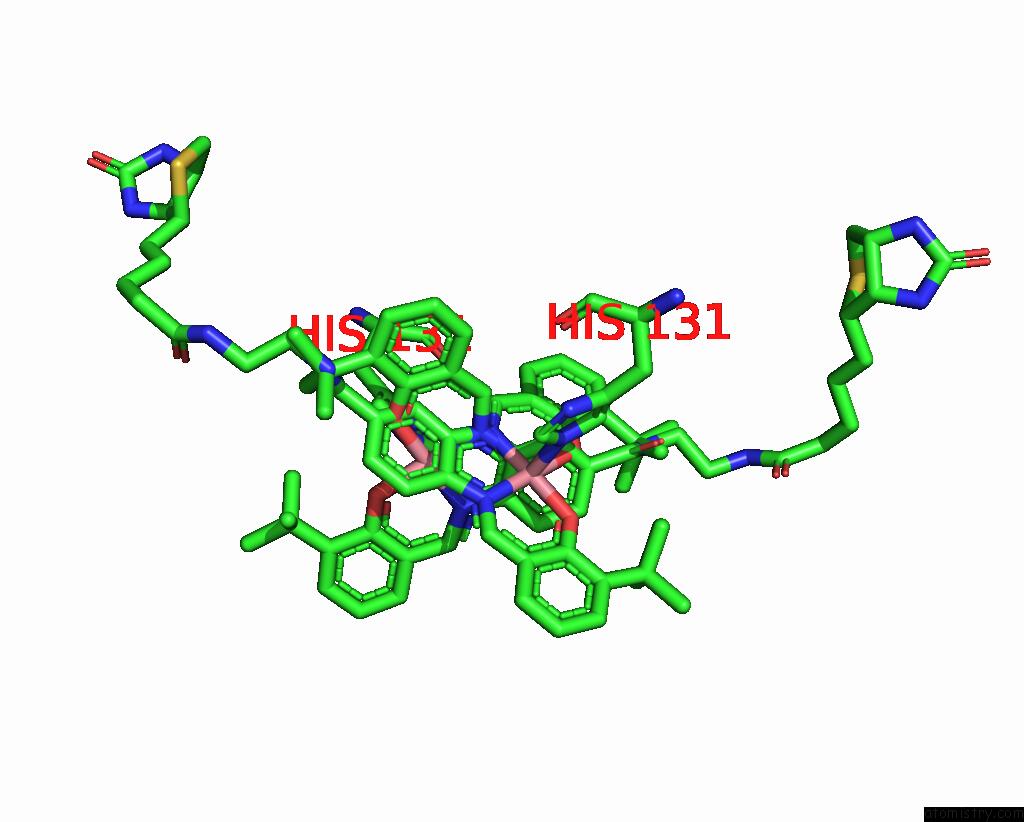
Mono view
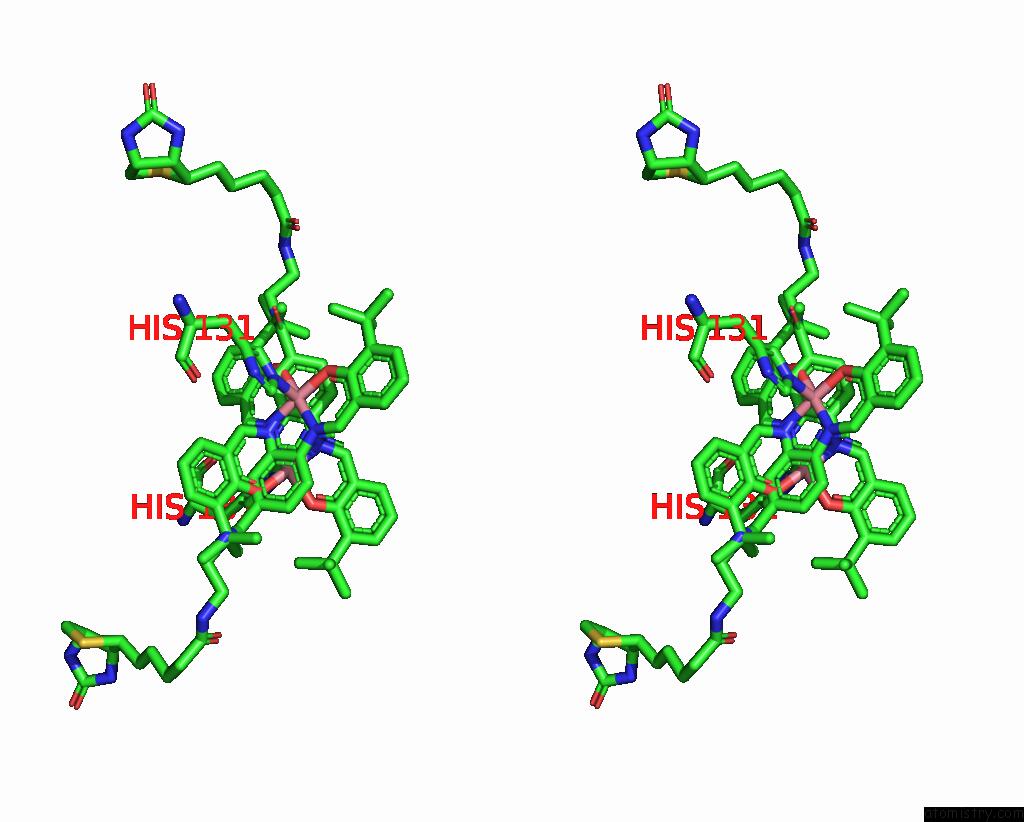
Stereo pair view

Mono view

Stereo pair view
A full contact list of Cobalt with other atoms in the Co binding
site number 2 of Streptavidin Variant with A Cobalt Catalyst For Ch Metal-Catalyzed Hydrogen-Atom-Transfer (M-Hat) within 5.0Å range:
|
Reference:
D.Chen,
X.Zhang,
A.A.Vorobieva,
R.Tachibana,
A.Stein,
R.P.Jakob,
Z.Zou,
D.A.Graf,
A.Li,
T.Maier,
B.E.Correia,
T.R.Ward.
An Evolved Artificial Radical Cyclase Enables the Construction of Bicyclic Terpenoid Scaffolds Via An H-Atom Transfer Pathway. Nat.Chem. 2024.
ISSN: ESSN 1755-4349
PubMed: 39030420
DOI: 10.1038/S41557-024-01562-5
Page generated: Sun Jul 13 22:03:42 2025
ISSN: ESSN 1755-4349
PubMed: 39030420
DOI: 10.1038/S41557-024-01562-5
Last articles
Cu in 6SJICu in 6S07
Cu in 6RYX
Cu in 6RYW
Cu in 6RYV
Cu in 6RRQ
Cu in 6RW7
Cu in 6RRP
Cu in 6RKZ
Cu in 6RIL